SEPTEMBER 19TH, 2016
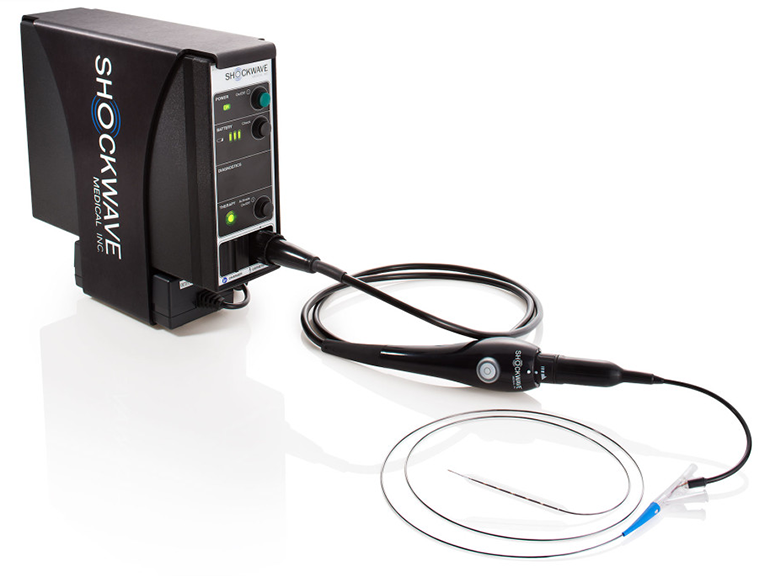
A unique system that uses a balloon and sound waves to break up plaque in patients with peripheral artery disease has just been cleared by the FDA. The Lithoplasty system from Shockwave, a firm out of Fremont, California, is basically a traditional angioplasty balloon catheter with added capability that resembles lithotripsy that’s used to break up kidney stones.
The transducers along the length of the balloon section are tuned to generate sound at frequencies that resonate hardened calcium. Being all shook up, the calcium deposits are motivated to crack and to allow the balloon to push them closer to the vessel wall. This results in a wider lumen and should lead to improved outcomes for patients with peripheral artery disease for whom an extra millimeter or two of increased space for blood to flow can make a whole lot of difference.
“Lithoplasty represents a new mechanism of treatment and is revolutionary for the care of patients with calcified peripheral vascular disease, a difficult-to-treat patient population,” in a statement said Kenneth Rosenfield, M.D., Section Head for Vascular Medicine and Intervention at Massachusetts General Hospital. “Existing devices for treating these patients have significant shortcomings that make it challenging to successfully open arteries, while minimizing vascular injury and complications. Lithoplasty is a unique approach that allows us to successfully treat these diseased vessels using a device built on a familiar balloon catheter platform, while minimizing the risk of vessel injury, including dissections that require stenting or other additional interventions.”