If peripheral vascular disease (PAD) is not treated, it can progress toward nearly complete blockage of the arteries that supply the lower extremity with blood and result in critical limb ischemia (CLI). People suffering with the condition experience severe pain, can have gangrene, and have a drastically reduced quality of life. A new device from LimFlow, a company based in Paris, France received the European CE mark to introduce a system that can offer a new option for otherwise untreatable patients.
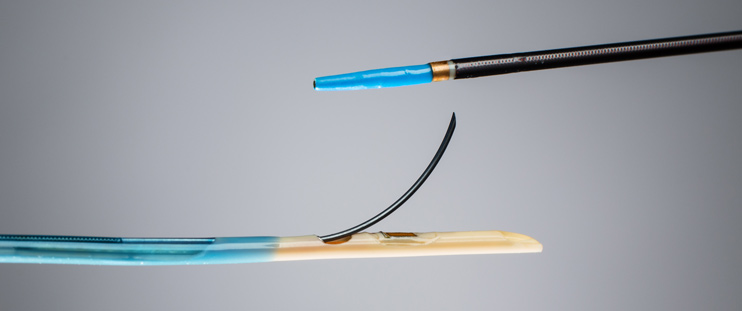
The LimFlow system is used to link the tibial vein and a diseased tibial artery of the leg so that blood can bypass the arterial occlusion and reach the foot. It relies on two catheters that utilize ultrasound to accurately line up next to each other. Once positioned, a guidewire from the arterial side is used to penetrate into the vein and to then place a covered stent that bridges the vessels. The stent is quite long, continuing toward the foot to provide the necessary support for all the new blood flow.
While not a miracle cure, as the patient will now have blood moving down the vein in the wrong direction, it may prevent terrible consequences such as amputation. “Utilizing the existing alternative pathway of the venous vasculature, the LimFlow System is designed to reestablish perfusion for patients that have chronic, non-healing wounds and are in imminent danger of losing a limb,” said Dan Rose, chief executive officer of LimFlow, in a statement. “We can now provide an option for patients that have none today. In early clinical cases, we have seen patients with extensive and severe foot wounds, including gangrene, fully heal following treatment with the LimFlow therapy, becoming mobile and active again.”