There are several topical corticosteroid formulations (including combinations with anti-infectives) that are registered and approved for use in Nigeria. The TCS formulations are listed by their generic names only. Brand details and detailed therapeutic classifications can be found in EMDEX Print or Mobile.
Choosing topical corticosteroids (TCS)
Topical corticosteroids are widely used for a variety of inflammatory skin disorders. They suppress the inflammatory reaction and relieve symptoms, but their actions are not curative and symptoms can recur on discontinuation of therapy.
Safe and effective use of TCS requires careful consideration of the following:
- Accurate diagnosis.
- Choice of steroid preparation, relative potency and delivery vehicle.
- Frequency of application and duration of treatment
- Potential adverse effects, both local and systemic
Appropriate indications
Topical corticosteroids are generally indicated for symptomatic relief of acute and chronic skin eruptions, where anti-inflammatory, anti-allergenic and antipruritic activity is required.
Indications for TCS include contact dermatitis, eczema (atopic dermatitis), psoriasis, insect bites; symptomatic relief for burning and pruritic lesions, etc. See below for detailed listing of skin conditions that may be responsive to topical steroid therapy.
TCSs should be avoided in untreated tubercular, bacterial and fungal infections involving the skin and in certain viral diseases such as herpes simplex, chickenpox, and vaccinia due to concerns that immunosuppression may exacerbate the infection.
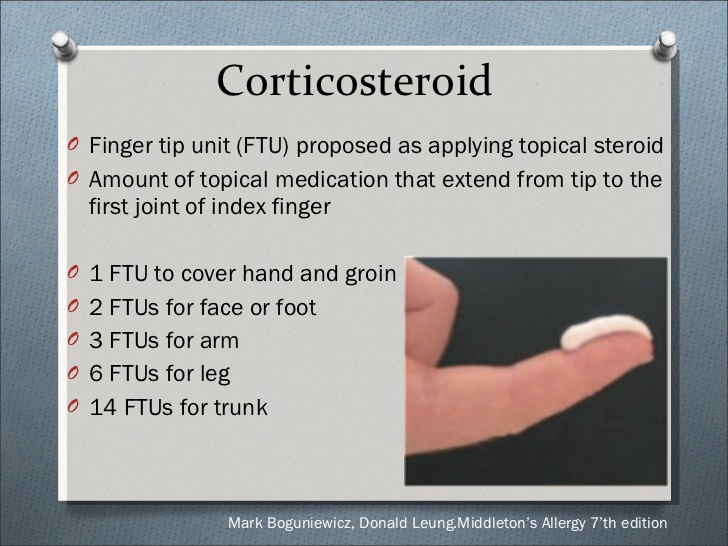
Dosing, frequency of application, and duration of treatment: “The Fingertip Unit Method”
A fingertip is from the very end of the finger to the first crease in the finger. The “Fingertip unit” (FTU) is a validated method of applying topical drugs in suitable safe quantities. One FTU is approximately 0.5 g, and is defined as the amount of topical steroid that can be squeezed out from a tube (with a stand-ard 5 mm nozzle) along an adult’s fingertip i.e., from the very end of the finger to the first crease of an adult’s index finger.
The frequency of application may vary with the product used and the condition being treated. Once or twice-daily application is recommended for most preparations. More frequent application is usually not needed, since the stratum corneum acts as a reservoir for these lipophilic compounds. An alter-nate day or even twice weekly application may be recommended in some chronic conditions due to this depot effect of TCS.
Duration of treatment for most conditions should not exceed 2-4 weeks, regardless of the potency of the TCS. Chronic application of topical steroids can induce tolerance and tachyphylaxis. High-potency steroids should not be used for more than three weeks continuously. If there is worsening of the le-sions or no change noticed, the product needs to be discontinued and re-evaluation of the diagnosis is indicated.
Selecting a vehicle
Topical corticosteroid preparations consist of an active ingredient and a vehicle (or solvent). In addition to being a carrier for the corticosteroid, the vehicle affects potency based on alterations of the steroid release rate and bioavailability.
The selection of vehicle depends on the type of lesions and the anatomical region. Some vehicles should be used only in certain parts of the body. Most topical corticosteroid preparations are available in several forms, including ointments, creams, gels, aerosols and lotions.
Ointments usually contain petrolatum, waxes, paraffin, propylene glycol, or mineral oil.
Preferred vehicle for Palm and Soles; nonhairy skin and also for dry or thick, hyperkeratotic lesions.
Most occlusive, provides better steroid absorption/penetration. Atopic skin conditions due to hydrating nature. Not suitable for hairy and intertriginous areas
Creams (oil-in-water emulsions) have good lubricating qualities and their ability to vanish into the skin make them cosmetically appealing.
May be used in any area of the body. Preferred vehicle for wet or weepy (exudative) lesions due to their drying effect; also for between skin folds.
Good lubricating and vanishing property, and cosmetic appeal. Generally, less potent than ointments. Suitable for use in intertriginous areas unlike ointments
Gels and lotions are the least greasy and occlusive of all topical steroid vehicles.
Gels may be used on the scalp and other hairy skin areas due to their drying & cooling qualities. Jelly-like effect makes it suitable for exudative inflammation (e.g., poison ivy) and for acne. Non-greasy and non-occlusive.
Lotions/Solutions contain alcohol, which has a drying effect on an oozing (weeping) lesion.
Preferred vehicles for the scalp and other hairy skin areas; between skin folds; moist, macerated lesions.
Easy to apply. Non-greasy and non-occlusive. Least potent topical therapies. Drying and cooling effects.
Topical corticosteroids, Combinations with antibacterials and/or antifungals
TCS combination with an anti-infective agent is indicated where the risk of infection is high or where there is an expectation that potentially dangerous numbers of bacteria or fungi will be present on the skin. The use of these combination products should be limited as some of the components can be sensitizing (e.g., Neomycin). These formulations are generally overused and sometimes, for cosmetic reasons. They allow for treatment without proper diagnosis and should be discouraged.
Recommendations for optimizing the use of topical corticosteroids
- Children generally require a shorter duration of treatment and a lower potency steroid.
- Prescribe for the right dermatoses, not as empiric therapy for every “rash”.
- Use appropriate steroid formulation and potency to achieve disease control.
- Initiate maintenance therapy with a lower potency steroid after achieving control of the acute inflammation.
- Taper off the treatment upon complete remission of skin diseases, after prolonged therapy.
- Limit the duration of use
- Caution when prescribing topical steroid for certain body regions (e.g., groin, face, axillae, and flexures).
- To be aware of the adverse effects and act immediately to counteract them.
- When infections necessitate the addition of an antibiotic or antifungal, systemic treatment should be considered.
- When the diagnosis is unclear or the condition is nonresponsive to standard treatment, refer to a dermatologist.
Comparison of topical corticosteroids
Ultra-high and High Potency TCS
Recommended for thick skin areas like Palm and Soles for: Atopic dermatitis (resistant); Discoid lupus; Hyperkeratotic eczema; Lichen planus; Lichen sclerosus (skin); Psoriasis; Severe hand eczema.
High-potency may also be used on the Trunk, Extremities, Scalp & Hairy skin areas for: Scalp dermatitis; Atopic dermatitis; Psoriasis, etc.
They pose the highest risk of systemic side effects. Avoid abrupt discontinuation; continuous daily use >3 weeks, and occlusive dressings.
Monitor for symptoms of adrenal suppression: weakness, weight loss, hypotension, and gastrointestinal distress.
Lower potency agents are preferred for the face, groin, armpits, or skin folds due to susceptibility to local side effects and systemic absorption
Ultra-high potency includes: Betamethasone dipropionate glycol (augmented) 0.05% Cream, Ointment, Lotion; Clobetasol 17-propionate 0.05% Cream, Ointment, Lotion; Halobetasol propionate 0.05% Ointment.
High potency includes: Amcinonide 0.1% Ointment, Cream, Lotion; Betamethasone dipropionate 0.05% Ointment, Cream, Lotion; Betamethasone valerate 0.1% Ointment; Fluocinonide 0.05% Cream, Ointment, Gel; Halobetasol propionate 0.05% Cream; Mometasone furoate 0.1% Ointment.
Moderate Potency TCS
Recommended for Trunk, Extremities, Scalp & Hairy skin areas for: Alopecia areata; Atopic dermatitis; Contact dermatitis (severe); Lichen sclerosus (vulva); Nummular eczema; Perianal inflammation (severe); Scabies (after scabicide); Seborrheic dermatitis; Severe dermatitis; Severe intertrigo (short-term); Stasis dermatitis.
Moderate to low potency agents should be used when treatment involves large body surface area.
Duration of treatment: May be used for up to 3 months when treating non-facial or non-intertriginous areas.
Occlusive dressings should be avoided.
Low Potency TCS
Recommended for thin skin areas like Face, Neck, Intertriginous or Genital areas for: Dermatitis (face, eyelids, diaper region); Intertrigo; Perianal inflammation.
These are agents of choice for children, pregnant women, elderly or for treating large areas.
Available TCS Combinations with Antibiotics
- Betamethasone + Neomycin (Topical)
- Hydrocortisone + Gentamicin (Topical)
- Hydrocortisone + Neomycin (Topical)
Available TCS Combinations with Antifungals
- Beclometasone + Clotrimazole
- Betamethasone + Clotrimazole
- Clobetasol + Clotrimazole
- Dexamethasone + Clotrimazole
- Diflucortolone + Isoconazole
- Hydrocortisone + Miconazole (Topical)
Available TCS Combinations with Antibiotics and Antifungals
- Beclometasone + Clotrimazole + Gentamicin
- Beclometasone + Clotrimazole + Gentamicin + Clioquinol
- Betamethasone + Clotrimazole + Gentamicin
- Betamethasone + Clotrimazole + Neomycin
- Betamethasone + Tolnaftate + Gentamicin
- Betamethasone + Tolnaftate + Gentamicin + Clioquinol
- Betamethasone + Tolnaftate + Neomycin + Clioquinol
- Clobetasol + Ketoconazole + Neomycin
- Clobetasol + Miconazole + Gentamicin
- Clobetasone + Miconazole + Gentamicin
- Dexamethasone + Clotrimazole + Gentamicin
- Dexamethasone + Clotrimazole + Neomycin
- Dexamethasone + Miconazole + Neomycin
- Fluocinolone + Miconazole + Neomycin
- Miconazole + Beclometasone + Neomycin
- Miconazole + Clobetasol + Neomycin
- Triamcinolone + Econazole + Gentamicin
Adverse effects of topical corticosteroids (TCS)
Risk factors for adverse effects
- Duration of treatment — long-term treatment is likely to result in systemic absorption.
- Area of the skin being treated — treating large areas of skin increases the risk of absorption.
- Condition of the skin — absorption is greatest in thin, inflamed skin.
- Potency of the topical corticosteroid — the greater the potency, the greater the risk of absorption.
- Occlusion — use of topical corticosteroids under occlusion increases the risk of systemic absorption.
- Age — children and elderly people are more susceptible to the adverse effects of topical corticosteroids because they have a thinner epidermis.
Local adverse effects
Common and mostly occur on the face, in skin folds, and in areas that are treated over the long term
- Transient burning or stinging — this is common, especially in the first 2 days of application on untreated, inflamed skin.
- Worsening and spreading of untreated infection.
- Thinning of the skin (atrophy) — the skin improves over a period after stopping treatment.
- Permanent stretch marks or striae. Most common in the groin, axillae, and inner thigh areas.
- Allergic contact dermatitis — due to the corticosteroid or the excipients. Lower potency agents like Hydrocortisone may be more allergenic.
- Acne (or worsening of existing acne) or rosacea. Common in the face with high potency agents.
- Hypopigmentation — More common in the blacks. May be reversible.
- Excessive hair growth at the site of application (hypertrichosis). Reversible.
Systemic adverse effects
Rare, but may occur more frequently in the presence of risk factors including infants and children
- Adrenal suppression.
- Cushing’s syndrome.
- Hyperglycaemia
- Growth retardation in children.
Minimizing adverse effects
- Prescribe the least potent formulation which is fully effective (advise the person to apply it thinly to affected areas, no more than twice daily).
- Consider prescribing an appropriate quantity of an emollient for use alongside the topical corticosteroid (for moisturizing purposes).
- Avoid prescribing potent corticosteroids for use on the face.
- Unless under specialist supervision, the use of potent (such as betamethasone valerate 0.1%) and very potent (such as clobetasol propionate 0.1%) topical corticosteroids, should be limited to: Use for up to 2 weeks, and No more than 50 g each week; Once–daily application is usually sufficient — maximum of twice daily.
- Avoid using occlusive dressings with topical corticosteroids (especially on large areas of the body).
- If a topical corticosteroid is needed for maintenance therapy, consider incorporating regular periods when they are withdrawn (for as long as possible) and emollients are used on their own.
- If the person is using large amounts of topical corticosteroid regularly, monitor them for signs of systemic adverse effects (such as adrenal suppression) and local adverse effects (such as areas of thin skin or striae).
- Monitor the height of children who are using large amounts of topical corticosteroid.
References
Available on request