C17.174.401
NO CONSENT
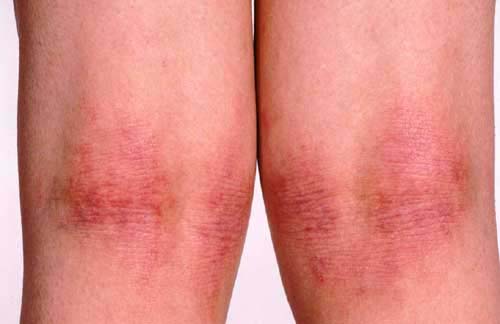
Most children’s eczema does not have a clear cause, such as an allergy, but most eczema will improve with good skin care. These tips from dermatologists can reduce the severity and frequency of your child’s flare-ups.
Bathing tips
- Bathe your child in warm — not hot — water.
- Limit your child’s time in the bath to 5 or 10 minutes.
- Use cleanser only when needed and make sure the cleanser is mild and fragrance-free. Do not use bubble bath.
- If your child’s eczema is frequently infected, twice-weekly bleach baths may be beneficial. Discuss this option with your child’s dermatologist.
- After bathing, gently pat your child’s skin partially dry.
- If your child has medicine that you apply to the skin, apply medicine when your child’s skin is almost dry and use the medicine as directed.
- Apply moisturizer on top of the medicine and to the rest of your child’s skin.
Tips for choosing a moisturizer
- When selecting a moisturizer, consider choosing a thick cream or ointment.
- Some children do better with fragrance-free products, so consider petroleum jelly — an inexpensive, fragrance-free product that works well for many children.
- When selecting a product, “trial and error” sampling of different types may help to identify the best moisturizer for your child.
Tips to ease discomfort
- For best results, apply moisturizer at least twice a day. This prevents dryness and cracking. It also can decrease the need for eczema medications.
- If your child has severe itching and scratching, ask your child’s dermatologist about wet wrap therapy. This can reduce swelling and lessen the desire to scratch.
- Keep your child’s fingernails short and smooth. This decreases the likelihood that scratching will puncture the skin. Putting cotton gloves on your child’s hands at night may help prevent scratching during sleep.
- Keep temperature and humidity levels comfortable. Avoid situations in which the air is extremely dry, or where your child may sweat and overheat. This is the most common trigger of the itch/scratch cycle.
Clothes-washing tips
- Using a laundry detergent made for sensitive skin may be beneficial. Scented fabric softener or dryer sheets may contribute to irritation.
- Only use the recommended amount of detergent.
- Use enough water for adequate rinsing.
- Buy clothes without tags because tags can rub against the skin, causing irritation.
- Wash your child’s new clothes before wearing. This will remove excess dyes and fabric finishers, which can irritate the skin.
Good skin care is a key part of gaining control of your child’s eczema. If skin care has not been a regular part of your child’s treatment, you should make an appointment for your child to see a dermatologist.