Duzallo was approved by the U.S. Food and Drug Administration (FDA) as a once-daily oral treatment for hyperuricemia associated with gout in patients who have not achieved target serum uric acid (sUA) levels with a medically appropriate daily dose of allopurinol alone. Duzallo is not recommended for the treatment of asymptomatic hyperuricemia. Ironwood expects Duzallo to be commercially available early in the fourth quarter of 2017.
Duzallo is the first drug that combines the current standard of care for the treatment of hyperuricemia associated with gout, allopurinol, with the most recent FDA-approved treatment for this condition, lesinurad. This fixed-dose combination provides a dual mechanism of action in a single tablet that can address both underlying causes of hyperuricemia – overproduction and underexcretion of serum uric acid.
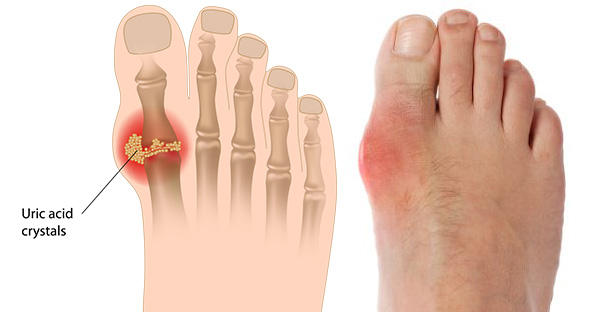
Gout is a highly symptomatic and painful form of inflammatory arthritis caused by hyperuricemia, or elevated sUA levels in the blood, which can lead to painful flares and serious potential long-term health consequences.
“The approval of Duzallo provides a new fixed-dose and dual-mechanism treatment option to help patients with uncontrolled gout achieve target serum uric acid levels. This represents an important and needed new option in the treatment of hyperuricemia,” said Michael A. Becker, M.D., professor emeritus of medicine, Department of Medicine, The University of Chicago, Chicago, IL. “Gout is a serious and potentially progressive and debilitating inflammatory disease. Getting patients with gout to serum urate goal, and keeping them at or below goal, are essential to success in treating these patients. Duzallo will help reduce the significant unmet need among patients in the U.S. who fail to get their serum uric acid levels to goal despite taking allopurinol alone.”
“With Duzallo, nearly twice as many patients with uncontrolled gout may be able to achieve target serum uric acid levels compared to those patients taking allopurinol alone, which is important, considering the significant unmet need among uncontrolled gout patients to get to goal of under 6 mg/dL,” said Tom McCourt, senior vice president of marketing and sales and chief commercial officer at Ironwood.
Duzallo (lesinurad and allopurinol) is a once-daily oral therapy that contains lesinurad 200 mg plus allopurinol 300 mg; it is also available in a lesinurad 200 mg plus allopurinol 200 mg dosage. Duzallo is approved by the FDA as a once-daily oral treatment for hyperuricemia associated with gout in patients who have not achieved target serum uric acid (sUA) levels with a medically appropriate daily dose of allopurinol alone. Duzallo is not recommended for the treatment of asymptomatic hyperuricemia. Allopurinol is an XOI whose action differs from that of uricosuric agents such as lesinurad. Allopurinol reduces the production of uric acid (UA); lesinurad increases renal excretion of UA by selectively inhibiting the action of URAT1, the UA transporter responsible for the majority of renal UA reabsorption. The dual-mechanism combination of Duzallo can address both inefficient excretion and overproduction of UA, thereby lowering sUA levels. Duzallo should be taken in the morning with food and water, and patients should be advised to stay well hydrated when taking Duzallo (about 2 liters of liquid a day).